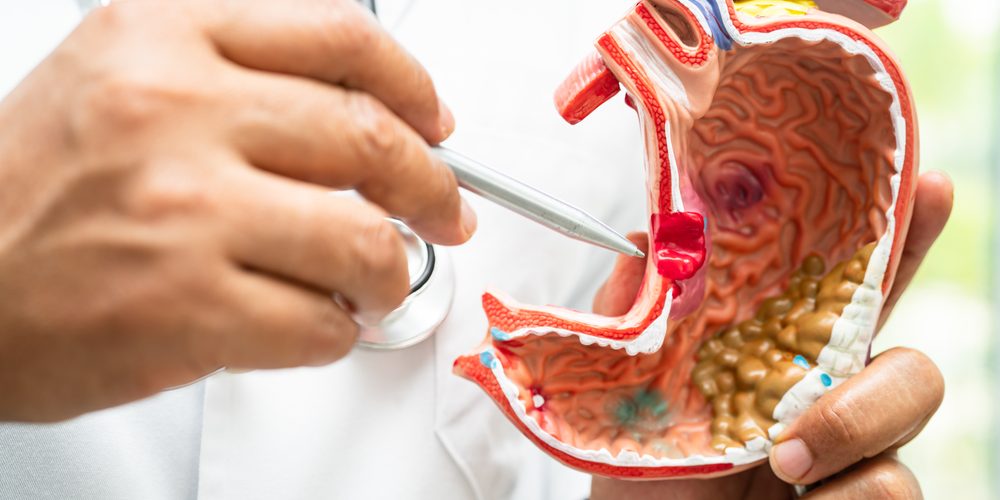
EMA accepts Astellas’ MAA for zolbetuximab to treat gastric cancer
Astellas Pharma Inc. announced the European Medicines Agency (EMA) has accepted for regulatory review the company’s marketing authorization application (MAA) for zolbetuximab, a first-in-class investigational Claudin 18.2 (CLDN18.2)-targeted monoclonal antibody, for first-line treatment of patients with locally advanced unresectable or metastatic HER2-negative gastric or gastroesophageal junction (GEJ) adenocarcinoma whose tumours are CLDN18.2-positive. If approved, zolbetuximab would be the first CLDN18.2-targeted therapy available in Europe for these patients.
Gastric cancer accounted for 3.1% of all new cancer cases in Europe in 2020, with around 136,000 new cases diagnosed. The average five-year survival rate for patients with gastric cancer in Europe is 26% across all stages.
“Patients with gastric cancer in Europe face extremely low five-year survival rates regardless of their disease stage, and innovative therapies that extend survival are needed,” said Moitreyee Chatterjee-Kishore, PhD, MBA, senior vice president and head of immuno-oncology development, Astellas. “The EMA’s acceptance of the zolbetuximab MAA continues a cascade of regulatory milestones for Astellas that are aimed at bringing a new option to patients with advanced gastric and GEJ cancer.”






![Moet u uw redactionele kalender weggooien ten gunste van deze marketingstrategie? [Video]](https://www.pharmamarketeer.nl/wp-content/uploads/2025/02/13792-80x60.jpg)

