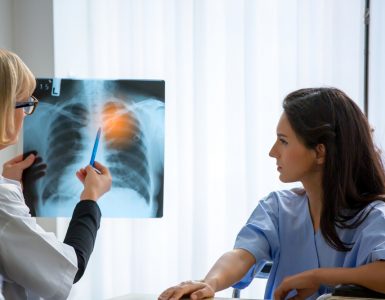
US firms Pfizer and Eli Lilly have said that NGF inhibitor tanezumab met its primary endpoint in a phase 3 trial of patients with moderate to severe chronic low back pain (CLBP).
Treatment with 10mg of tanezumab significantly improved pain at 16 weeks when compared to placebo.
An estimated 33 million Americans suffer from CLBP, eight million of whom suffer from a moderate-to-severe form of the condition. CLBP is currently the leading form of disability. Meanwhile current treatments do not meet many patients’ needs.